Applications of
Quantum Mechanics
Quantum Mechanics
Implications of
Quantum Mechanics
Quantum Mechanics
34A. Quantum Mechanical Explanation of Scattering.
Summary
In a scattering experiment, the wave function hits many grains of film. But quantum mechanics shows we perceive only one grain as exposed.
In a scattering experiment, the wave function hits all the grains on the ‘detector’ surrounding the target particle, but only one grain is found to be exposed. Surprisingly, this exposure of only one localized grain by a spread-out wave function can be accounted for strictly from the principles of quantum mechanics, without invoking the existence of particles. It depends on the linearity of the theory.
We suppose in the electron scattering example that there is an interaction V(gr,i) between the electron wave function and each of the N grains,
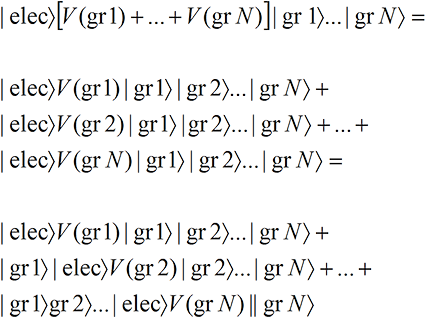
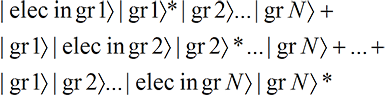
We have assumed that in each term, the electron—or rather a portion of the electron-like wave function—ends up lodged in the exposed grain. Note that the small portion of the electron-like wave function that lodges in each grain carries the full Mass, Spin and Charge that we associate with an electron so that each of the N versions of reality has the correct charge and spin.That is, there are N terms and only one grain is exposed in each term. But we know from Classical Perception that only one term will be perceived. And since each term has one and only one localized grain exposed (no matter what the size of the grains!), only one localized grain will be perceived as exposed, even though the electron wave function hits all the grains! Thus a spread-out wave function leads to perceptually localized effects.
As we said before (in Localization), it is remarkable that quantum mechanics alone, with no assumption of the existence of particles, can imitate, in our perceptions, the classical idea of a localized (only one localized grain exposed) particle.

