Applications of
Quantum Mechanics
Quantum Mechanics
Implications of
Quantum Mechanics
Quantum Mechanics
33. Spin and the Stern-Gerlach Experiment.
Summary
The Stern-Gerlach experiment, used to determine the discrete values of spin in quantum mechanics, is described. This conceptually important experiment nicely illustrates the many-versions-of-reality idea.
Discrete values of angular momentum, or spin as it is called on the atomic level, are one of the hallmarks of quantum mechanics. And the Stern-Gerlach experiment, used to measure spin, is one of the most-used examples for illustrating ideas in quantum mechanics. So it is useful to gather all the relevant ideas in one place.
The invariance chain of reasoning. As we noted in Mass, Spin and Charge, rotations of the whole apparatus do not affect the outcome of experiments. This implies the equations must have the same form in all rotated coordinate systems. This form invariance in turn implies that solutions of the equation can be labeled by spin or angular momentum. And we further find that the spin, even though it is initially just a label on a solution, can actually be measured, so that the labels have physical meaning. That is, we have this chain of reasoning—from physical invariance (rotations shouldn’t matter in the outcome) to mathematical invariance to a mathematical classification scheme for different wave functions to an actual, physical, measurable property of the wave function.
Classical and quantum angular momentum. Angular momentum was defined in classical physics centuries ago; it is the ‘momentum around the center’ (momentum times the radius) a ‘particle’ has when traveling in a circle. This could take on any value, so that classically, angular momentum is a continuous variable.
But that is not true in quantum mechanics. Instead, when measured along a certain direction, angular momentum (spin) can only take on certain discrete values. For a spin 1 wave function, the allowed values (in units of the Planck constant
The Stern-Gerlach experiment. Angular momentum or spin is measured on an atomic scale by the Stern-Gerlach experiment. A particle (particle-like wave function) is shot into a magnetic field and the field exerts different forces on the parts of the wave functions having different values of spin. Because of the differing forces, the parts of the wave function with different spin exit the magnetic field traveling in slightly different directions. Suppose, for example, that spin 1 particles are shot into the magnetic field. Then three different streams of particles will come out of the apparatus; the +1 spin stream might be traveling slightly upward, the spin 0 stream would travel straight through, and the -1 spin stream would be travelling slightly downward. Three different detectors could be put in the three paths to determine, on a single run, which path ‘the particle’ took (this ‘particle’ and ‘which path’ language is convenient but misleading).
Many versions of reality. So let’s suppose the wave function for the particle before it reaches the magnetic field is a linear combination of the three possible spins,
We now put a detector on each of the paths, and suppose there is an observer looking at the outcome (yes or no) displayed on each dial. The full wave function, particle plus detectors plus observer, is then the sum of three parts;
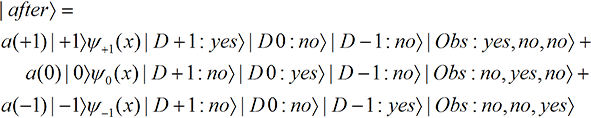
Spin
Interestingly, the abstract group representational theory (with ‘abstract’ here meaning not associated with a particular problem) allows the observed spin

