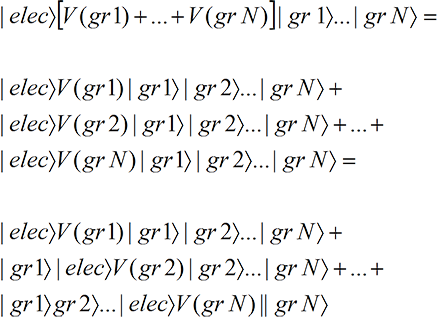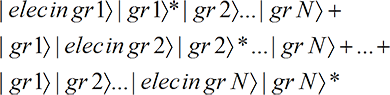Principles and Concepts
of Quantum Mechanics
Implications of
Quantum Mechanics
12A. Details of Localization.
Summary
The rules of quantum mechanics imply only one localized grain of film is perceived as exposed by a wave function spread out over many grains. Details.
We suppose in the electron scattering example that there is an interaction, represented by
V in the equations below, of the electron wave function,
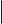 elec
elec
, with each of the N grains,
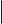 gr
gr
. Just before the wave function hits the grains, we can schematically write the state and electron-grain interaction as
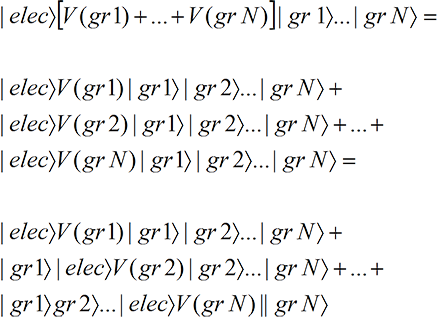

(12A-1)
where we have used allowable rules of re-arrangement of the N terms to arrive at the final form. In the first term of the last three lines, the electron wave function, through the grain 1 interaction term, interacts with and exposes only grain 1. In the second, only grain 2 is exposed, …, and in the last term, only grain N is exposed. Thus the final state is (with the asterisk indicating an exposed grain)
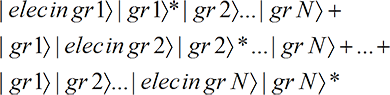

(12A-2)
[We have assumed that in each term, the electron—or rather a portion of the electron-like wave function—ends up lodged in the exposed grain. Note that the small portion of the electron-like wave function that lodges in each grain carries the full mass, spin and charge that we associate with an electron—see
Mass, Spin, and Charge—so that each of the N versions of reality has the correct spin and charge.]
That is, there are N terms and only one grain is exposed in each term. But we know from
Quantum Mechanics and Classical Perception that only one term will be perceived. And since each term has just one grain exposed (no matter what the size of the grains), only one
localized grain will be perceived as exposed, even though the electron wave function hits all the grains!